articles
Dec 22, 2024
The FDA’s New Definition of "Healthy"
What It Means for Food Labeling and CPG Founders
In a landmark update, the FDA has redefined the term "healthy" for food labeling, marking the first major change since 1994. The new rule reflects modern nutrition science, focusing on dietary patterns and food groups instead of individual nutrients. This update could have significant implications for the Consumer Packaged Goods (CPG) industry, particularly for founders navigating reformulation, labeling, and consumer preferences.

Here's a deep dive into the new rule and how it could impact your brand
What’s New in the FDA’s “Healthy” Definition?
The FDA’s updated rule prioritizes food groups and dietary patterns over individual nutrients, aiming to provide consumers with clearer guidance about food choices. Here are the key updates:
New Focus on Food Groups (Instead of Individual Nutrients)
Previous Rule (1994): The “healthy” claim was based on limits for individual nutrients, such as fat, saturated fat, cholesterol, sodium, and minimum amounts of certain nutrients like vitamin A, vitamin C, calcium, iron, protein, or dietary fiber.
NEW RULE (2024): Foods must now meet specific thresholds for food group equivalents (FGEs). These FGEs are based on serving sizes that align with the Dietary Guidelines for Americans, 2020–2025, which emphasize whole dietary patterns over isolated nutrients.
Food Group Equivalents (FGEs) Introduced
The rule defines specific qualifying amounts of food groups to meet the "healthy" claim:
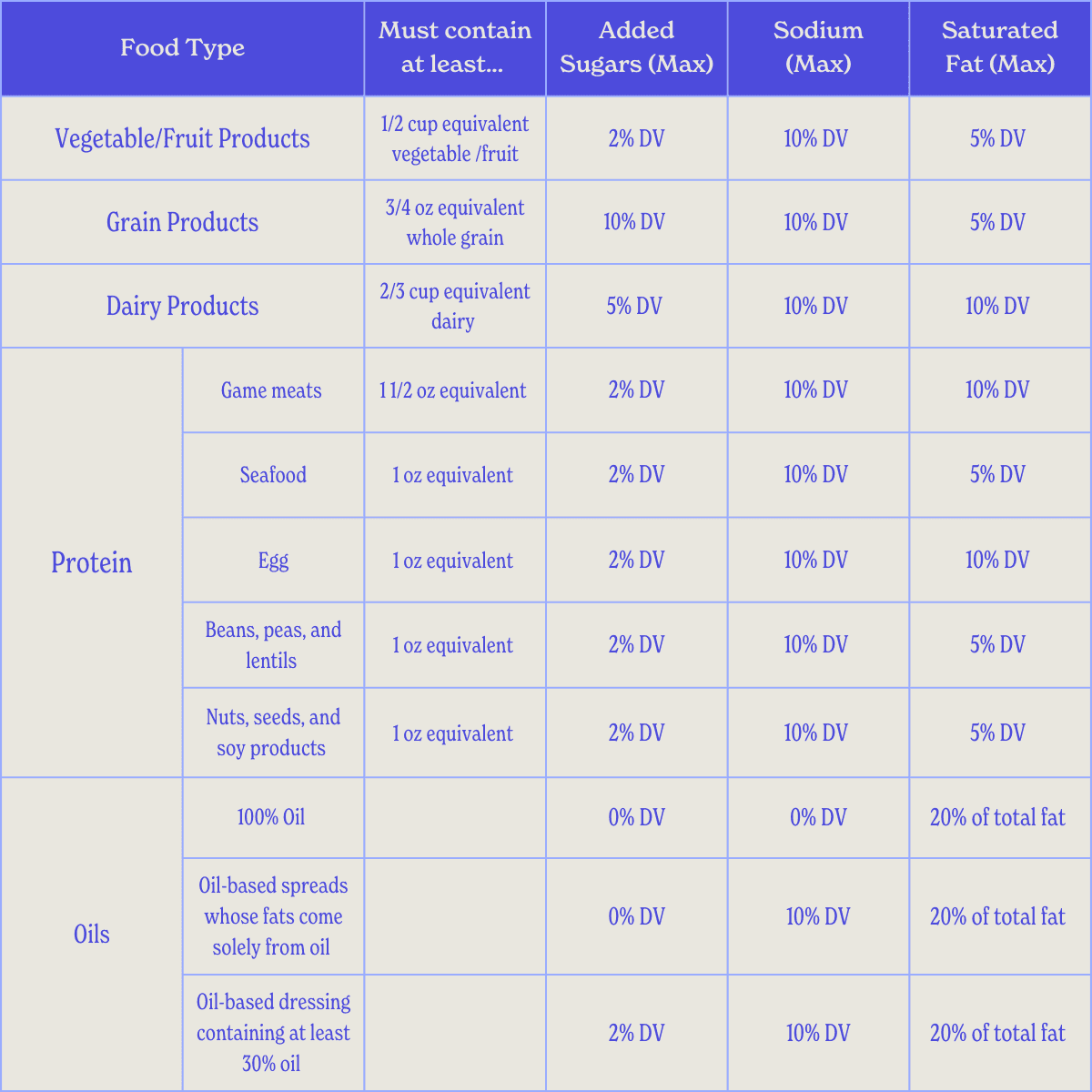
Vegetables: At least ½ cup equivalent (c-eq) per serving.
Fruits: At least ½ cup equivalent (c-eq) per serving.
Grains: At least ¾ ounce (oz) equivalent whole grain.
Dairy: At least ⅔ cup equivalent (c-eq).
Protein Foods:
Game meat: 1½ oz equivalent.
Seafood: 1 oz equivalent.
Egg: 1 oz equivalent.
Beans, peas, lentils: 1 oz equivalent.
Nuts, seeds, soy products: 1 oz equivalent.
Oils: 100% oil qualifies, or oil-based spreads/dressings meeting thresholds.
Thresholds for Nutrients to Limit (NTL)
Foods must stay within limits for added sugars, sodium, and saturated fats as a percentage of the Daily Value (DV). These thresholds vary by category:
Automatic Qualification for Certain Foods
Some foods automatically qualify as “healthy” without meeting FGEs and NTL limits:
Raw, whole vegetables, fruits, and whole grains (with no added ingredients except water).
Plain water, tea, and coffee (with fewer than 5 calories per serving).
Lean meat, seafood, eggs, beans, peas, lentils, nuts, and seeds (without added ingredients).
Expanded Allowances for Packaged Foods
The new rule allows packaged, frozen, canned, and shelf-stable products to qualify as "healthy" if they meet the nutrient and food group requirements. This inclusion ensures that affordable, accessible, and culturally preferred foods can bear the claim.
Adjustments for Smaller Serving Sizes
For foods with a Reference Amount Customarily Consumed (RACC) of 50 grams or less (e.g., snack items):
The FGE and nutrient thresholds are calculated per 50 grams, instead of per serving size. This adjustment ensures that smaller-portion foods like certain snacks and spreads can qualify as “healthy.”
Flexibility for Combination Foods
The rule introduces a flexible framework for mixed products, main dishes, and meals:
Mixed Products: Must contain 1 total FGE, with no less than ¼ FGE from at least two food groups.
Main Dishes: Must contain 2 FGEs, with no less than ½ FGE from at least two food groups.
Meals: Must contain 3 FGEs, with no less than ½ FGE from at least three food groups.
Exemptions for Certain Ingredients
Saturated Fat in Seafood, Nuts, Seeds, and Soy: Saturated fat inherently present in these items is excluded from the NTL threshold, recognizing their predominantly beneficial fat profiles.
Vegetable and Fruit Powders: These can now contribute to FGEs, as they retain the same nutrient density as their whole forms.
Recordkeeping Requirements
Foods using the "healthy" label must maintain records for two years to verify compliance with FGEs and nutrient thresholds. The FDA may request these records during inspections.
Compliance Timeline
The rule will take effect 60 days after publication, but brands have until February 25, 2028 to achieve full compliance.
What Stayed the Same?
The "healthy" claim remains voluntary, and brands that do not meet these new criteria can still promote other nutritional attributes via alternative nutrient content or health claims (e.g., "low sodium," "good source of fiber").
These updates are designed to reduce consumer confusion, align with modern dietary guidelines, and encourage the development of nutrient-dense food products across categories.
How will this impact CPG founders?
Reformulation Challenges
Products that previously qualified as “healthy” may no longer meet the updated criteria, particularly if they contain high levels of added sugars, sodium, or saturated fats.
Founders will need to reformulate recipes to align with the new thresholds while maintaining taste and texture.
Labeling and Packaging Updates
Any product marketed as “healthy” will require updated packaging and documentation to prove compliance. The compliance date is set for February 25, 2028, giving companies time to prepare.
Marketing Opportunities
Meeting the new “healthy” standards can differentiate your product in a crowded market, especially as consumers prioritize transparency and better-for-you options.
Highlighting compliance with the updated definition can improve brand trust and appeal to health-conscious shoppers.
Broadening Product Scope
The inclusivity of frozen, shelf-stable, and culturally diverse foods opens doors for brands targeting multicultural markets or offering more accessible price points.
Plant-based products, snack-sized portions, and nutrient-dense combinations now have a clearer path to meeting “healthy” standards.
Consumer Perception
The revised definition shifts focus from avoiding “unhealthy” nutrients to incorporating nutrient-dense food groups, helping brands better align with modern consumer values around health and wellness.
The Bottom Line
The FDA’s updated “healthy” definition represents a major shift in how foods are evaluated and marketed. By emphasizing nutrient-dense food groups and modern dietary patterns, the new rule aligns with evolving consumer expectations and public health goals. For CPG founders, this is an opportunity to innovate, reformulate, and lead in the better-for-you product space.
The challenge is clear, but so is the reward: a healthier product portfolio that resonates with consumers and strengthens your brand in a health-conscious marketplace.
Check out the full ruling HERE!